The European Union Health Technology Assessment Regulation (EU HTAR) 201/2282 is reshaping the evaluation process for pharmaceutical products across the EU. As manufacturers navigate this transformation, their preparedness for the EU Joint Clinical Assessment (JCA) remains a critical question. To gain insight into industry readiness, our EU HTA experts conducted in-depth interviews with key industry leaders.
The EU JCA will reshape the pricing and market access (P&MA) landscape across Europe, creating both opportunities and challenges for pharmaceutical companies. As of January 12, 2025, oncology products and ATMPs seeking EMA marketing authorization must undergo this assessment, with orphan drugs expected to follow in 2028 and full implementation for all medicines by 2030.
In this article, we assess how pharmaceutical companies are preparing for the EU JCA, examining the extent to which they feel equipped to navigate this landscape and how they are adjusting their P&MA strategies in response to this change. Drawing on in-depth interviews with 12 senior P&MA leaders from small and large pharmaceutical companies, we evaluate the perceived opportunities and challenges posed by the EU JCA and outline best practices to help pharmaceutical companies achieve EU JCA readiness.
Manufacturers recognize potential opportunities associated with the EU JCA
It is difficult to see the benefits right now. There are many challenges and few answers. In the long-term though, the benefits may outweigh the challenges, but only if the regulation is implemented appropriately, and if the manufacturer perspective is taken into account.
During discussions, industry experts highlighted three key opportunities that the EU JCA presents for pharmaceutical companies:
- Greater cross-functional collaboration
The new regulation establishes a new “customer” who drives a unified evaluation process that significantly influences access to health technologies across the EU. With EU HTA being closely linked to regulatory approval and local HTA processes, it demands more integrated, cross-functional collaboration—an opportunity that manufacturers recognize may lead to improved internal processes and outcomes. This shift also positions Market Access and HEOR teams to take the lead in strategy preparation and ensure the cross-functional development of the EU HTA strategy.
- Improved access across the EU
EU HTA may also improve patient access by having a single clinical assessment that may be leveraged by all EU member states. Availability of the EU JCA report, ideally within 30 days after regulatory approval, means that EU payers will be able to initiate their local P&R processes without conducting a local clinical assessment. Over time, deferring to the EU clinical assessment may accelerate reimbursement timelines where resource constraints often delay local assessments. By enabling a more uniform approach to clinical evidence evaluation, the EU JCA is expected to facilitate faster and more equitable access to innovative therapies across Europe, supporting both patient and industry objectives.
Over time, we do expect that the EU HTA regulation can improve patient access, in particular in terms of time to access in markets beyond EU-4.
- Potential for long-term efficiency gains
The EU HTA can also drive long-term efficiency gains in how companies generate and submit evidence for HTA purposes. Over time, manufacturers may be able to consolidate their efforts and focus on developing the EU HTA dossier instead of 27 national dossiers currently required in the EU. However, these efficiency gains hinge on local payer acceptance of the EU JCA reports, which may eliminate the need to submit comprehensive national dossiers in the long run.
Manufacturer enthusiasm is tempered by a variety of foreseeable challenges
There is potential, but for now, this is rather theoretical, with lots of uncertainties for us.
Despite potential opportunities, manufacturers remain cautious about the challenges posed by the EU HTA. Overall, industry experts perceive challenges in three key areas:
- Procedural uncertainties
One perceived challenge is the lack of clarity surrounding the EU HTA. While Implementing Acts and guidance documents have been published, the framework is still evolving and many questions remain on how implementation will actually work as no products have gone through the process. Although recent publication of EUnetHTA PICO exercises provided early insights, uncertainties remain—particularly regarding the number of consolidated PICOs manufacturers can expect to be requested to address. Case studies of three medicines have shown a wide range, from 7 to 13 PICOs, raising concerns about the actual number of PICOs that companies should be planning for and that will ultimately be requested.
What is a PICO?
PICO is a framework guiding Joint Clinical Assessments (JCA) by defining the Patient population, Intervention, Comparator, and Outcomes. This framework standardizes the evaluation of clinical evidence across EU member states.
The Joint Scientific Consultation (JSC) process offers insights into the potential scope of EU JCA, but only a limited number of spots have been offered to manufacturers. Almost all manufacturers raised concerns regarding adequate resourcing for the JSC process, noting the best source for predicting PICOs will not be available to all manufacturers.
We are hoping to get a scientific consultation, however, it seems that there aren’t enough slots. Regulators should ensure equitable access to all manufacturers.
- Lack of clarity on real-life harmonization of local and EU HTA processes
While the EU JCA aims to harmonize clinical evaluations, most national payers have not yet published guidance on how it will be integrated locally. This uncertainty raises questions about potential delays in the reimbursement process and impact on launch sequencing.
Germany was the first country that released detailed guidance on how the JCA will be harmonized with the AMNOG process. However, this guidance has starkly evolved over time. While the possibility of postponing the start of the AMNOG process by 3 months was initially discussed, the actual regulation did not include such an allowance. Instead, if the JCA report is not available at the start of the AMNOG process, the manufacturer must provide the G-BA with their EU JCA dossier within three business days, and the JCA dossier will be used to initiate the local evaluation until the JCA report is available.
- Need for additional investment to ensure EU JCA readiness
Preparing for the EU HTA will require additional investment from manufacturers, ranging from additional resources to support internal preparation to more investment in evidence generation. Rather than replacing some of the requirements of national processes, manufacturers worry that the EU JCA will function as an added layer of assessment. As a result, they anticipate needing to dedicate more resources to ensure compliance with these new requirements alongside the existing national-level processes.
In addition, they are also concerned about the tight timelines in the EU HTA process. For instance, companies only have 100 days to complete the EU JCA dossier, 10 to 15 days to submit any missing information after JCA dossier submission, and less than a week to review the draft JCA report. Such tight deadlines demand greater internal coordination and faster decision-making. Beyond timeline challenges, manufacturers may also need to invest in additional evidence generation activities to meet EU JCA PICO requirements. Given the uncertainty in the number of PICOs that will be requested, manufacturers are compelled to invest in multiple additional studies to ensure they are ready for the EU JCA evaluation.
Right now, the whole process comes on top. We cannot yet shift resources as local processes remain at the core of pricing and reimbursement decisions.
Only around a third of pharmaceutical companies feel well prepared for EU JCA
While around two-thirds of manufacturers believe that they are at least somewhat prepared for the EU JCA, their current readiness appears to hinge on two factors: the scale of their organization and whether their near-term pipeline includes a drug in scope for the first wave of JCAs. As expected, organizations with assets subject to the EU JCA in the next 1-2 years have a higher level of readiness. They possess a profound understanding of available EU HTACG guidance, assess key strategic implications, and outline next steps for the company. Overall, larger organizations show a greater level of readiness compared to smaller companies and are already identifying and addressing their organizational refinement needs.

Large and small pharmaceutical companies without assets to undergo EU JCA in the next few years show a lower level of readiness and seem to adopt a “watch and wait” outlook. We also assessed whether other company characteristics such as geographic base were relevant in predicting the level of industry readiness. However, these do not seem to play a meaningful role. The fact that around two-thirds of companies feel only somewhat prepared for EU JCA highlights the need for additional guidance to help manufacturers successfully navigate the evolving EU JCA landscape. By the same token, it is imperative for companies to take a proactive approach to their EU HTA preparations.
Integrating the EU JCA process into organizational strategies is required to capitalize on opportunities
Drawing on the industry insights, we recommend manufacturers to focus on three dimensions to ensure EU JCA readiness:
- Strengthen cross-functional collaboration
To successfully navigate the EU JCA process and maximize its potential benefits, each function needs to adapt to the new EU HTA requirements.
Market Access will continue to play a critical role in coordinating the creation of an integrated access and EU HTA strategy, co-leading this initiative with the HEOR team. In addition, HEOR teams will play a crucial role in ensuring that evidence generation plans address the needs of both EU JCA and national HTA bodies. Regulatory Affairs teams must closely coordinate EMA submission timing with EU JCA requirements and work closely with the Market Access and HEOR teams on any label updates as this may change the scope of the EU JCA. Meanwhile, Medical and Clinical Development teams need to integrate EU JCA considerations into early clinical trial designs and ensure the targeted populations and comparators will support the eventual access and EU HTA strategy. Finally, Commercial functions need to assess how the EU JCA will impact market sequencing. Given the increased complexity brought by the EU JCA, a siloed approach between functions is no longer viable.
The new regulation really forces us to work together. We have always had an emphasis on cross-functional collaboration, but the need to collaborate has never been as tangible.
- Embed EU JCA requirements into strategic planning
The EU HTA requires an update of the launch readiness processes of companies and an embedding of EU JCA requirements into key governance approval decisions. The cornerstone of readiness for EU JCA is ensuring the organization has a thought-through PICO strategy in place, and data is being generated to support the anticipated PICOs that will be requested by the HTA CG.
A proactive approach to predicting and prioritizing PICOs is essential to align clinical development plans with EU JCA requirements. Starting this exercise prior to pivotal clinical trials and refining these predictions is key to anticipating potential evidence generation gaps and determining the feasibility of generating additional evidence. Although payers and manufacturers recognize inherent trade-offs in PICO evidence generation, pivotal trials and supporting evidence development plans should reflect the anticipated PICOs to be requested by the EU JCA.
Companies must also incorporate EU JCA considerations into their launch deliverables, ranging from early access strategies to Global Value Dossiers (GVDs). Most of the interviewed experts plan to update the GVD to incorporate EU JCA requirements, ensuring a consistent storyline of the product’s value strategy.
Over the past year, we have overhauled our planning processes as well as our global deliverables to ensure they meet EU JCA requirements.
- Develop a roadmap to guide internal and external activities
For a successful EU JCA, manufacturers must establish a roadmap aligning internal and external activities. The first step is appointing dedicated leads, either at above-brand or brand-level, and establishing a Center of Excellence to initially coordinate efforts across functions. In larger organizations, the EU JCA Lead heads a global task force, bringing together Market Access & Pricing, HEOR, Medical, Clinical Development, Regulatory Affairs, Legal, and affiliates from major markets.
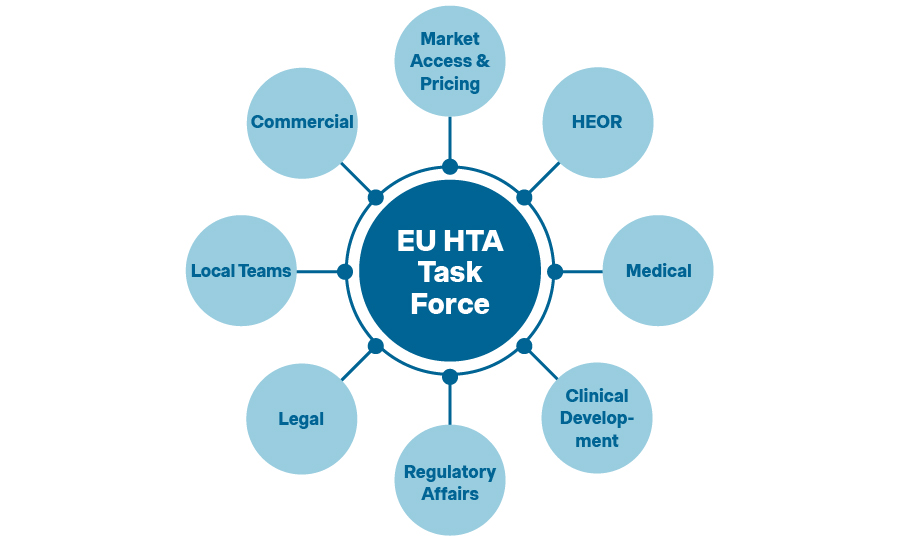
We appointed a new EU JCA Lead who heads a task force that brings together all relevant functions. You cannot adequately prepare for EU JCA without a dedicated team.
The most prepared companies are developing training curriculums to ensure all functions are aware of the EU HTA requirements. They are also creating workflows to share emerging developments at both EU and local levels across key functions and setting up agile governance processes to ensure fast decision-making, given the quickly evolving landscape.
Companies are also actively working with industry trade organizations to address process-related questions before their assessments begin. They are also raising policy and methodology concerns in hopes that the regulation will evolve with time.
Preparedness levels vary but pharma companies must be EU JCA-ready
Manufacturers remain at varying levels of preparedness and many have remaining questions on how to effectively prepare for the first round of EU JCAs. The challenges—ranging from procedural uncertainties to increased resource demands—underscore the importance of strategic planning, cross-functional collaboration, and early engagement with stakeholders.
Interested in learning more? Contact our experts today to discuss this in more detail.
Thanks to contributions from Jade Monteiro!