China’s volume-based procurement (VoBP) for pharmaceuticals has been broadening its reach and accelerating pace, with mounting pressure for pharma and medtech majors. Our experts outline actionable approaches to cope with the challenges.
When the going gets tough, the tough get going. The scope of China’s drug procurement policies is broadening. Called volume-based procurement (VoBP) these policies are unfolding at a faster pace and with greater price pressure. As such, pharma companies may need to consider a systematic approach, that would help them plan ahead and brace for imminent impacts.
VoBP policies have shaken up China’s pharma and medtech industries. Since 2018, there have been six rounds of national VoBP for 234 drugs with 53 percent price cuts on average, and two rounds for medical consumables targeting coronary stents and orthopedics implants at over 80 percent price cuts.
The seventh national VoBP is currently ongoing for another 58 drugs, many of which are blockbusters that have passed their exclusivities. This includes 25 injectables like octreotide, tirofiban, and zoledronic acid. At the same time, as we noted in our earlier PharmaExec piece Improving Readiness for Volume-Based Procurement in China, VoBPs at regional levels have been unfolding at a broader scope, faster pace, and with more price pressure. In fact, the National Health Security Administration recently announced one of its new year resolutions, which is to include 350 drugs and five categories of high value consumables in VoBP policies in 2022 for all provinces. They aim to achieve this through a combination of national and regional VoBPs.
VoBP policy impacts could be drastic, manifesting both in price and in volume, which often lead to double shocks overnight for established originators. Building on our extensive experience working with VoBPs, we’ve developed a proven approach to help businesses navigate through the whole VoBP process.
Simon-Kucher’s VoBP Trilogy®
As a Trilogy®, the approach consists of actionable steps to strategize before, simulate during, and rejuvenate after VoBPs.
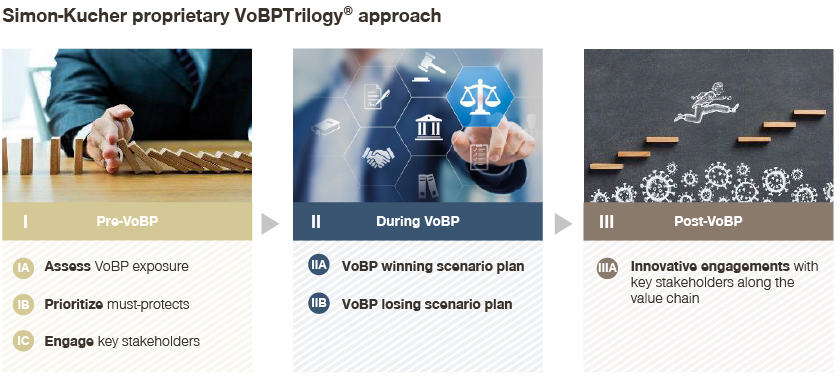
At the pre-VoBP stage, planning ahead is key. There are three crucial elements for effective planning and strategizing:
- Companies first need to have a thorough and objective baseline assessment of their VoBP exposures. Exposures come from both national VoBPs with higher visibility and impacts, as well as regional VoBPs which tend to be less predictable and may include those that have not lost their exclusivities. According to our assessment, within a three-year span VoBP exposures range from 60 to 90 percent for most multi-national pharma majors
- Pharma majors then need to identify the must-protect assets and regions, and that entails systematically assessing VoBP listing and tendering rules, mapping out cross-referencing implications, and tailoring internal preparations and external engagements to achieve must-wins
- Effective engagements with key stakeholders will be essential for policy shaping. This will help payers and key stakeholders balance budget impact considerations with drug quality requirements, and strive to ensure key factors like clinical differentiations, real world evidence, and outcomes, while the next round of VoBP rules and policies are still in the planning stages
At the VoBP tendering stage, the most important decision is whether to play the price game to win the tender, or opt out to keep some pricing latitude
To make the right decision, it’s important to have a good understanding of the tendering rules for that VoBP round. There have been both smart plays and gigantic fumbles when it comes to tendering. Some have done everything to win a tender but lost out when quoting one cent above the implied winning range. While in other cases the originators offered excessive cuts and outbid all generic competitors, leaving a huge amount on the table and everyone dumbfounded.
After analyzing the rules, it’s equally important know your business and team, as well as your opponents. For an originator with a dominant market share prior to VoBP, there is little to gain from winning a tender, as it will lead to steep price cuts from winning VoBP, but unlikely to garner gains in volume or market share.
For a generic player with limited share but cost advantage, the incentive for winning a VoBP tender is much stronger, as it may gain disproportionate shares in one fell swoop. It also saves resources and investment typically required for marketing, sales, and access as a new generic entrant.
In most cases though, the price-volume tradeoffs are less clear cut, and require scenario planning, competitive simulations, and even game theory from time to time, to ensure going into each tender with the right price or opting out for the right outcome.
Simon-Kucher’s 5P5E® model for GTM innovations
As well as making tactical preparations, pharma majors would benefit from a more strategic retooling of go-to-market (GTM) models to rejuvenate growth post VoBP. To that end, companies would need to have a holistic assessment of China’s healthcare value chain and its key stakeholders – namely peers, providers, pharmacies, patients, and payers.

Below are some best practice examples of companies applying the strategies we outlined in this article:
- Engaging with a medtech company – a diabetes major launched a new real time glucose monitoring solution for better diabetes management. The device provides health solutions by synergizing with the diabetes drug which experienced severe price cuts after a pyrrhic victory with national VoBP. This solution improves adherence and outcomes for the patients
- Enabling providers – a branded generics major expecting VoBP for its osteoporosis products partners with community clinics to offer preventative screening for senior residents. In so doing, the company supports primary care institutions improve diagnosis, treatment, and care standards for osteoporosis, building goodwill and brand equity directly with healthcare professionals (HCPs) and patients alike, ahead of VoBP
- Enhancing pharmacy presence – working closely with leading pharmacy chains and online pharmacies, a leading pharma doubled down with retail coverage and brand building, after opting out of VoBP for its cardiovascular blockbuster. While its hospital sales halved post-VoBP, retail channel sales grew by 77 percent the year after, which offset a significant portion of the aftermath
- Empowering patients – a neurology major partnered with online platforms JD Health and AliHealth for better disease education and health management. Its online portals afford patients with better disease knowledge, expert consultations, and end-to-end solutions. It does this through online prescriptions, refills, and deliveries, while capturing real-time customer insights based on big data. In the process, the company helps build intimate relationships and lifetime stickiness with patients and patient families
- Ensuring payers –a pharma major proactively established a unique clinical profile and differentiated value propositions of its key product. It was also successful in convincing payers to remove the product from VoBP scope in some provinces, steering itself into more favorable price negotiation processes in other provinces. In this way, it preempted VoBP price cuts and cross-region price referencing for the brand, to a large extent
While the strategic thrusts may look different for different assets and circumstances, the common theme is to tailor GTM models to address the unmet needs of key stakeholders, and to innovate and rejuvenate growth despite VoBPs.
As the going gets tough, the tough get going. So, it is more important than ever for pharma and medtech majors to plan, strategize, and innovate to thrive in the VoBP era.