China’s 2022 NRDL could be impacted by the latest omicron wave in a number of ways. Experts Bruce Liu, Ivy Jiang, and Miranda Wang outline the potential challenges ahead to help manufacturers prepare.
China came out of the COVID-19 pandemic in 2020 and 2021 largely unscathed but was caught by surprise in the latest omicron outbreaks in 2022. The accumulated COVID cases at 1.2 million by mid-May pale in comparison to most other countries, but the lockdowns have been draconian in many cities, with Shanghai among the hardest hit since late March.
Overreacting or not, China will feel the impact in many ways. The National Reimbursement Drug List (NRDL), as the major public funding pathway (updated annually), may face the ripple effects this year.
Potential delay of the NRDL timeline
Each year, the NRDL process normally takes around six months, starting in June, with results out in November. This is so it can be implemented starting from January of the following year.
Given the evolving omicron outbreaks in 2022, particularly impacting Shanghai and Beijing, this may delay the NRDL timeline. A similar situation occurred for the 2020 NRDL, when the Wuhan outbreak delayed the process by one month and implementation by three months.
Yet still, NRDL experts believe the process will take place and likely be wrapped up by end of 2022.

Increasing focus on public health
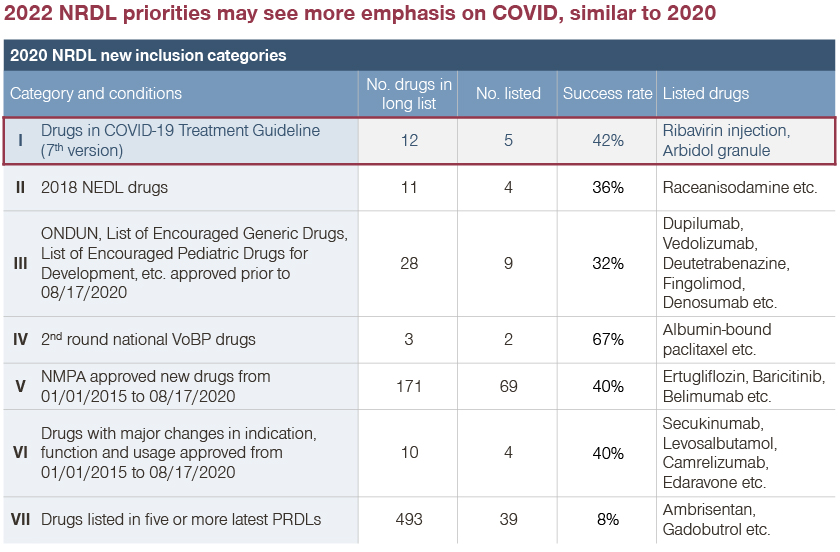
Since 2020, the Chinese National Health Commission (NHC) has published nine editions of its “Guideline for Diagnosis and Treatment of Coronavirus Disease”. On the basis of the guideline’s recommendation, many COVID-19 therapies have been included in the NRDL:
- In 2020, Ribavirin and Abidol were covered by the NRDL with recommendation in the seventh edition of the guideline
- In 2021, two traditional Chinese medicines approved in March 2021 and recommended by the guideline were included in the NRDL that same year
In the latest guideline, two new antiviral therapies – Paxlovid and Amubarvimab/Romlusevimab – have been recommended. It’s worth noting that these drugs have been temporarily included in provincial public reimbursement coverage right after the latest guideline was released.
Given the renewed emphasis on public health and COVID-19, new therapies could be prioritized for the 2022 NRDL, potentially as a separate and priority category.

Budge impact and price pressures
During the COVID outbreak, the NRDL’s income growth rate hit a historical low due to a phased exemption of basic medical insurance (BMI) contribution of urban workers and residents. BMI contributions this year may be impacted in a similar or more drastic way, due to lockdowns across multiple regions.
Meanwhile, high expenditures on vaccines and related testing for mass screening are also an extra financial burden to the BMI fund.
- According to the NHSA, over 120 billion Chinese Yuan (~18 billion US dollars) have been spent on vaccines by April 2022, co-funded by BMI and fiscal funding
- Current widely used antigen testing is also temporarily covered in public medical insurance across provinces
While the government has been striving to manage spending on the pandemic and refused to pay for PCR testing, the pressure on overall budgets is very likely to manifest in the price expectations and upcoming negotiations for the 2022 NRDL.
 ä
ä










